来源:宝创日期:2021-02-01(377)
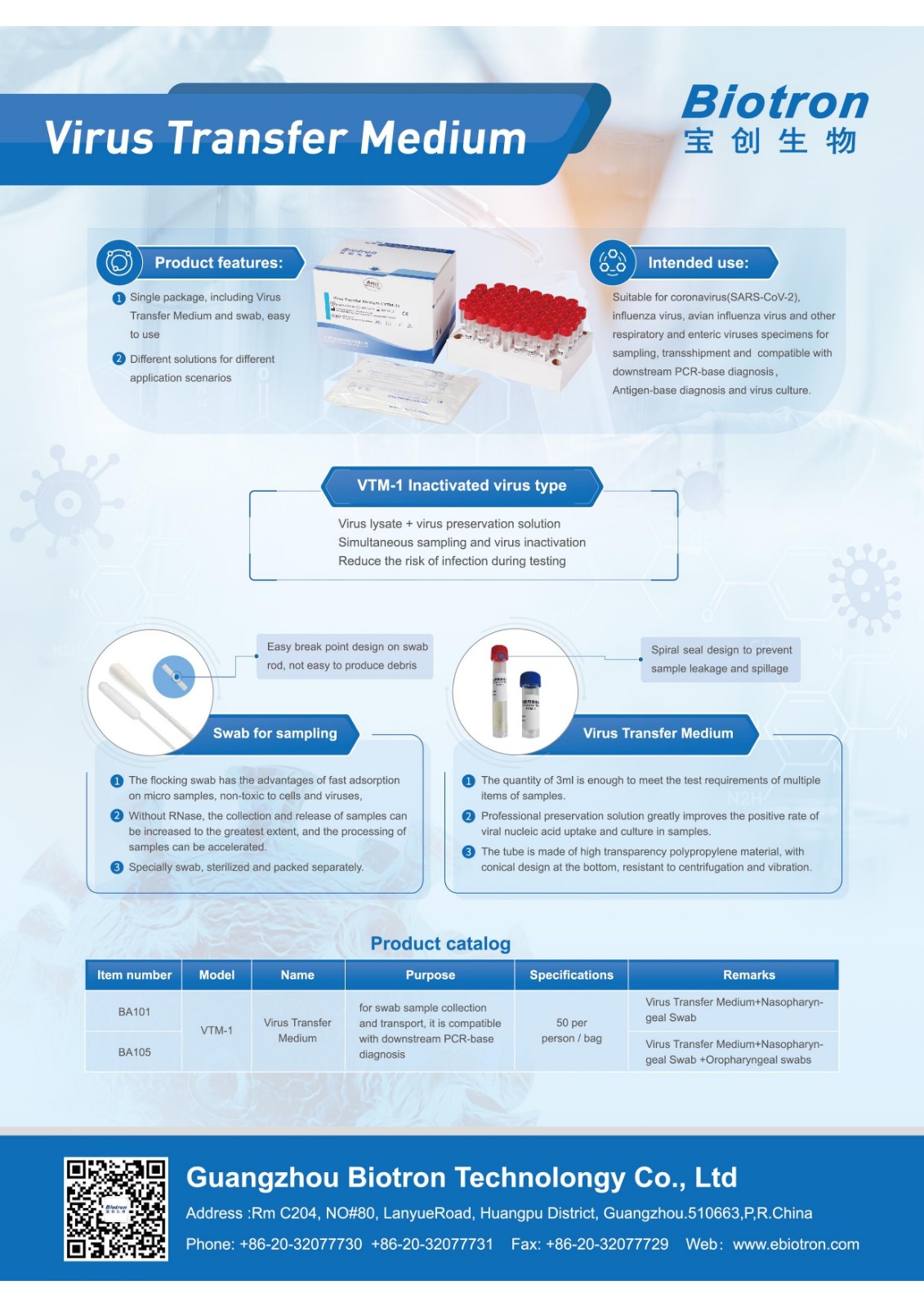
The new coronavirus is raging around the world. The nucleic acid detection of the new coronavirus is a key link in the prevention and control of the epidemic. How to efficiently and safely sample, transport, and detect nucleic acid has become a concern of frontline medical workers. Biotron has succeeded in developing antibodies and nucleic acids After the detection kit, it is committed to solving the difficulties in the initial stage of virus sampling, and launched a series of virus sampling/cultivation tubes, so as to realize the whole process and multi-faceted solution from sampling to detection.
Biotron’s efforts to help the global fight against the “epidemic” have never stopped. The new product VTM series virus sampling tube has successfully obtained the EU CE access qualification after obtaining the domestic filing certificate. According to the relevant EU laws and regulations and certification procedures, the products comply with the " Compliance requirements of the EU Medical Device Directives.


The video below is Virus transfer medium instruction for use