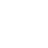
Lp-PLA2 Rapid Test (Fluorescence immunochromatography)
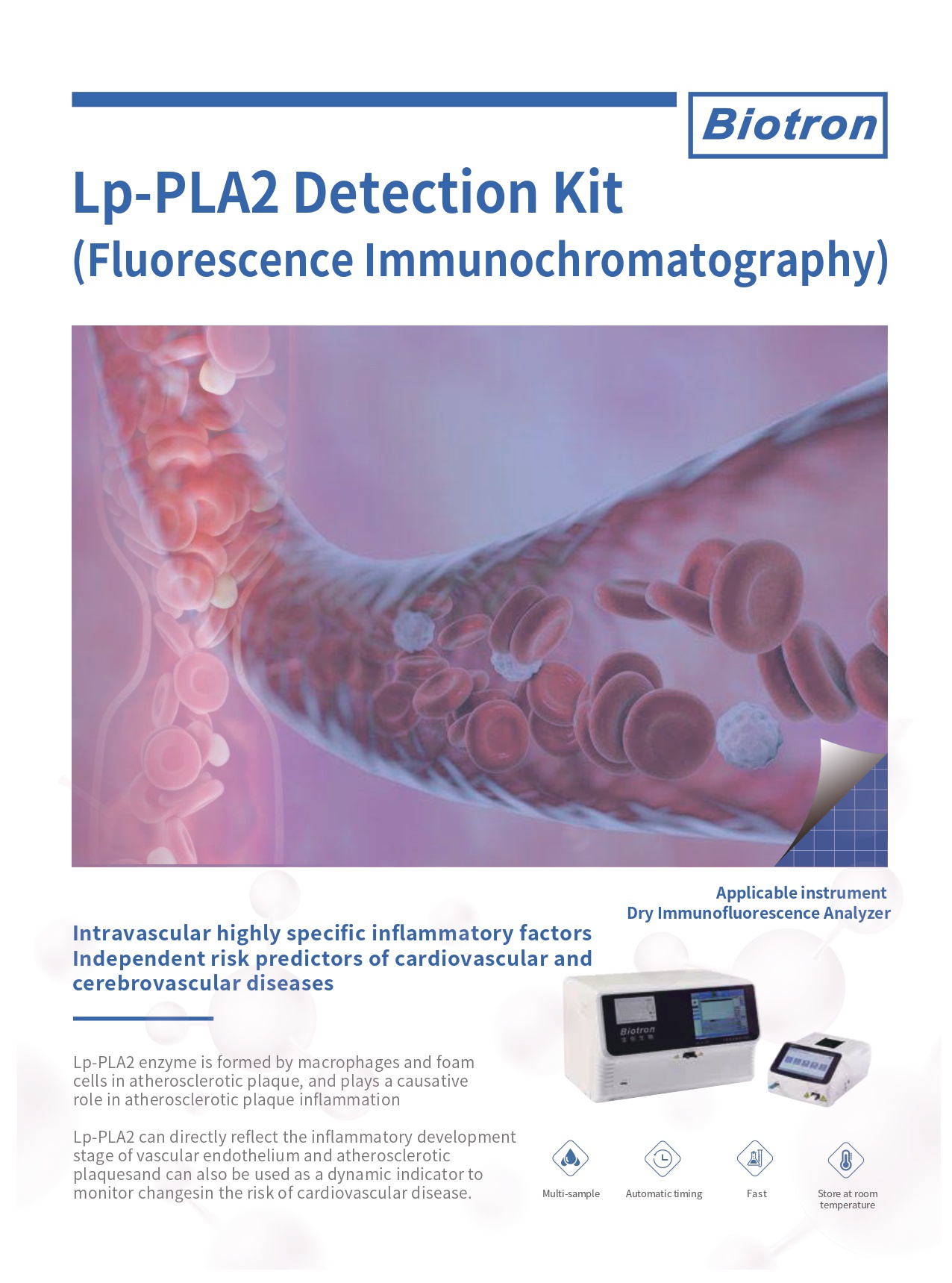
Lp-PLA2 enzyme is formed by macrophages and foam cells in atherosclerotic plaque, and plays a causative role in atherosclerotic plaque inflammation
Applicable to various sample types of serum, plasma and whole blood
Rapid detection of single reagent (15 minutes)
Convenient storage and transportation at room temperature
DESCRIPTION
Successfully obtained in the EU CE access certification
Passed ISO13485 certification
Lp-PLA2: an emerging biomarker of coronary heart disease
- Intravascular highly specific inflammatory factors
- Independent risk predictors of cardiovascular and cerebrovascular diseases
Lp-PLA2 induces an increase in plaque instability, making the plaque fragile and ruptured, leading to thrombosis and ischemic stroke. In addition, high levels of Lp-PLA2 predict increased instability of atherosclerotic plaques, more prone to rupture, and increased risk of cardiovascular and cerebrovascular malignant events.Schematic diagram of Lp-PLA2 is shown in Figure 1.
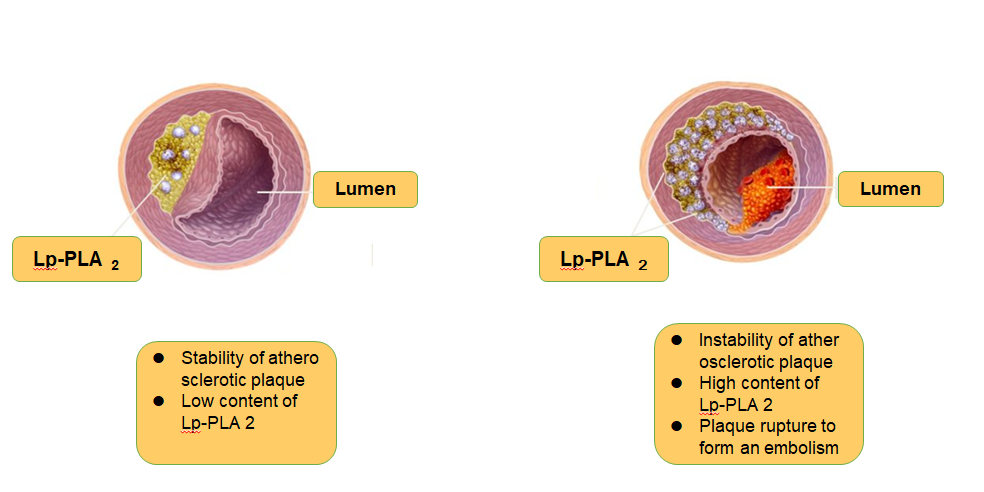
Figure 1
Schematic diagram of Lp-PLA2 and plaque stability
References:
The international recognition and importance of Lp-PLA2 is gradually increasing, and it is recommended that Lp-PLA2 be used in various aspects of cardiovascular and cerebrovascular diseases such as screening, differential diagnosis, risk assessment, treatment and prognosis.Guideline Recommendation Table is shown in Figure 2.
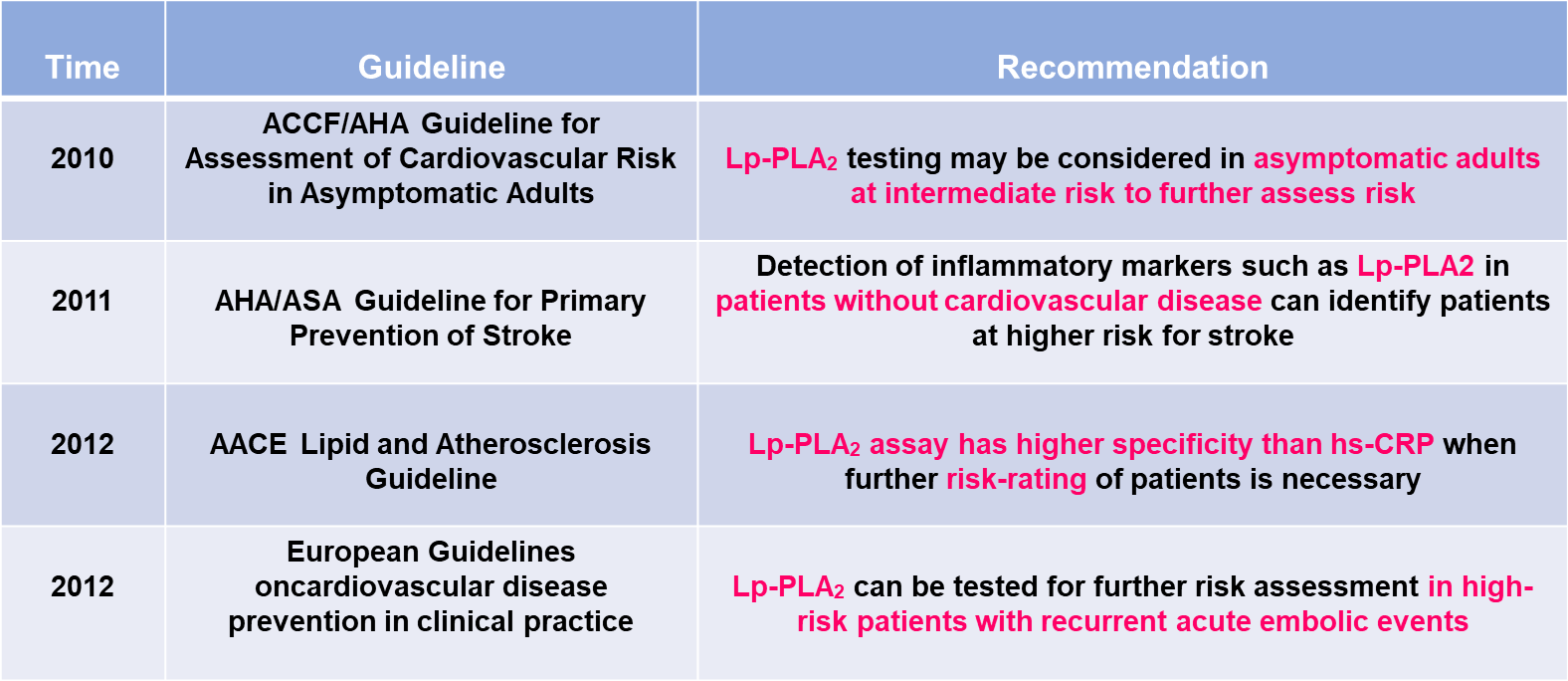
Figure 2
Lp-PLA2 Guideline Recommendation Table
Clinical significance:
-Dynamic monitor of the degree of inflammation in the vascular endothelium and atherosclerotic plaques
-Risk estimation for coronary heart disease and stroke
-Estimated risk of recurrence for various cardiovascular embolisms (stroke)
-Efficacy assessment , the decreased level of Lp-PLA2 is positively correlated with the lipid-lowering effect of statins
Applicable departments:
Cardiology, cardiac surgery, neurology, veteran department, endocrinology, laboratory department, physical examination center
SPECIFICATION
| Sample type | serum, plasma, whole blood |
| Report time | 15min |
| Reference range | |
| Storage | 4-30℃, sealed and kept away from light and dry |
| Validity period | 18 months |
| Specifications | 25 tests/box, 50 tests/box |

 Request
Request
Applicable Instrument

CATALOGS
 2 pages
2 pages