The Novel Coronavirus(2019-nCoV) Nucleic Acid test kit (RT-PCR)
The Novel Coronavirus(2019-nCoV)Nucleic Acid test kit is an in vitro real-time PCR-based test for the detection of Coronavirus in a single closed tube assay.
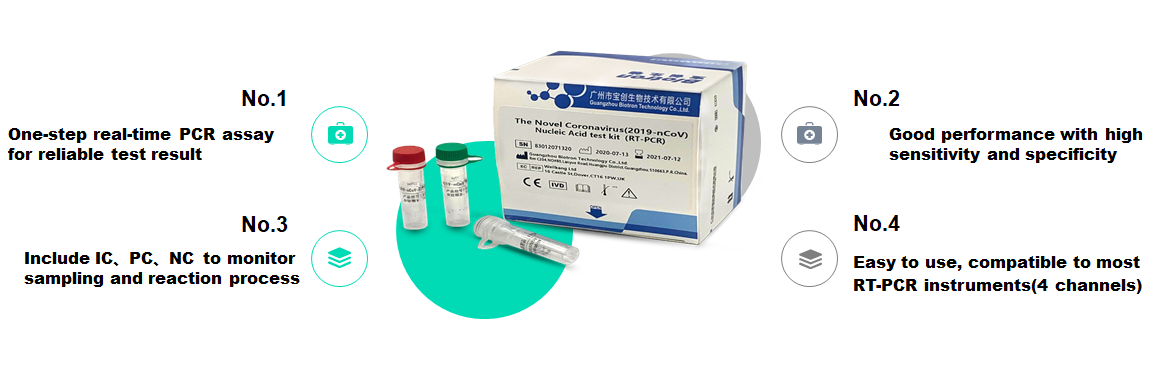
Reliable one-step real-time PCR detection results
High sensitivity and good specificity
Including IC, PC, NC to monitor the sampling and reaction process
Easy to use, compatible with most RT-PCR instruments (4 channels)
DESCRIPTION
Successfully obtained in the EU CE access certification
Passed ISO13485 certification
The 2019 novel coronavirus (2019-nCoV), is a contagious virus that causes respiratory infection and has shown evidence of human-to-human transmission, first identified by authorities, as the cause of coronavirus outbreak all over the world. Genomic sequencing has shown that it is a positive-sense, single-stranded RNA coronavirus. Rapid and accurate screening methods are highly desirable so that the correct treatment can be provided and to prevent further spread of such contagious infections.
The new coronavirus (2019 nCoV) nucleic acid detection kit is a single-closed test tube detection method for detecting coronavirus based on in vitro real-time PCR.
Product advantage
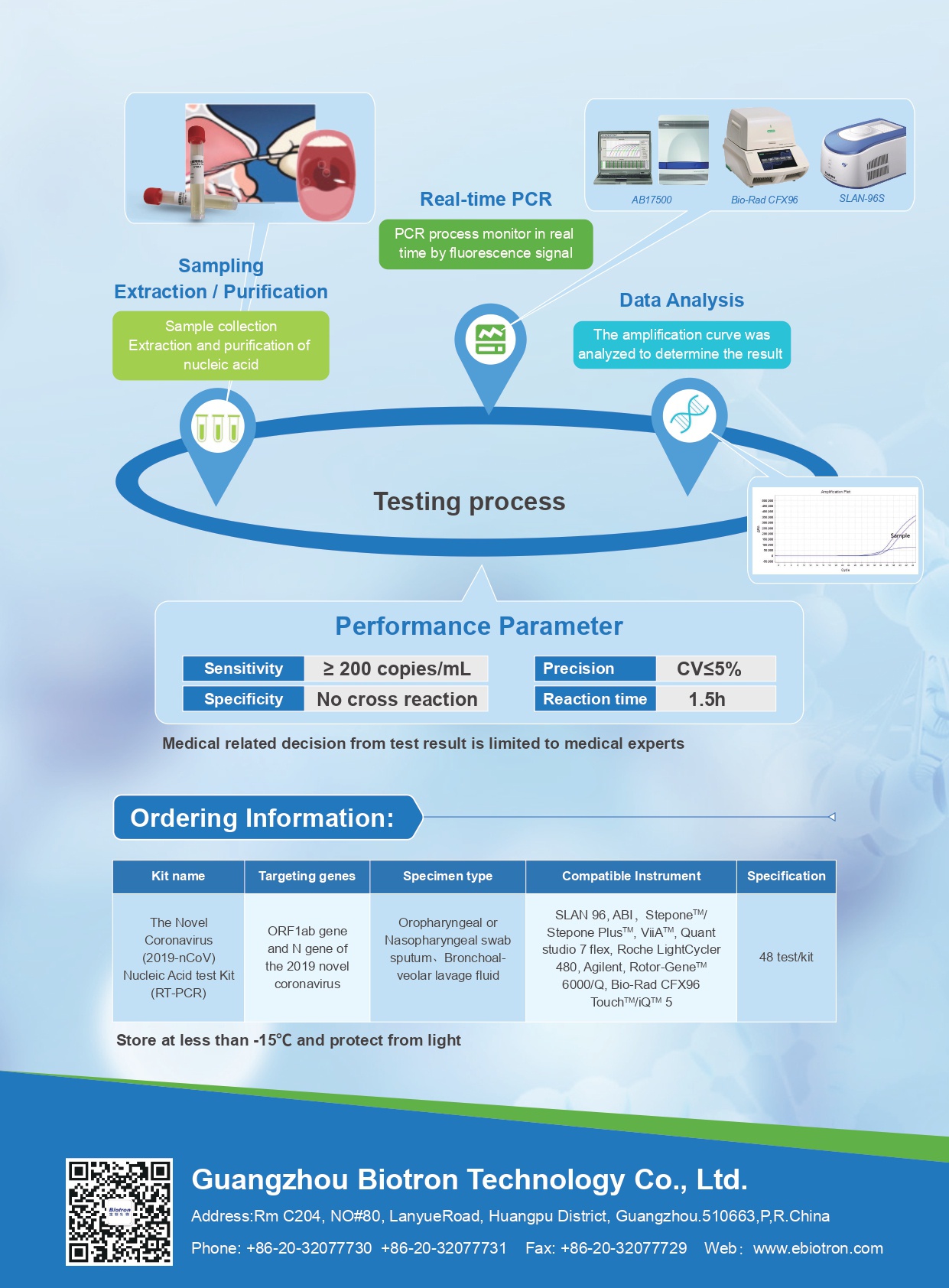
Test procedure
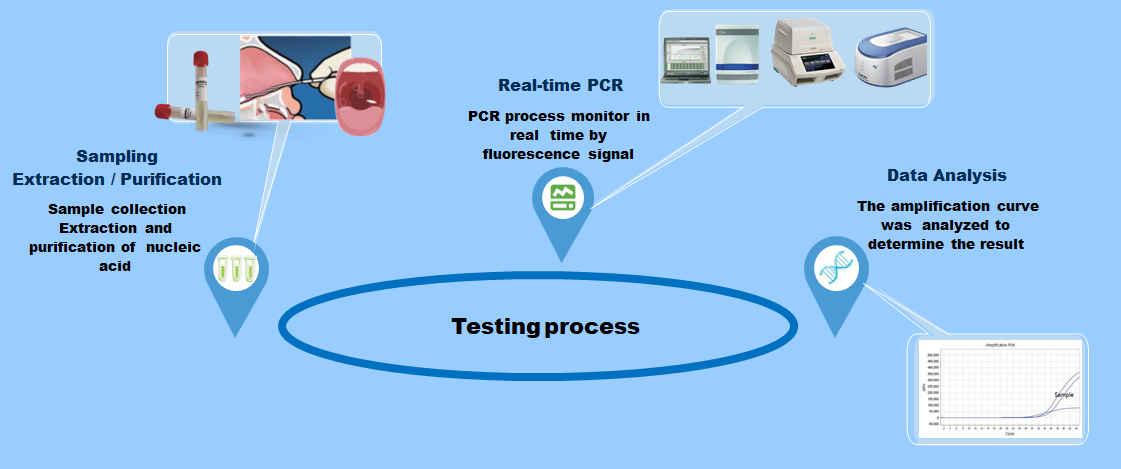
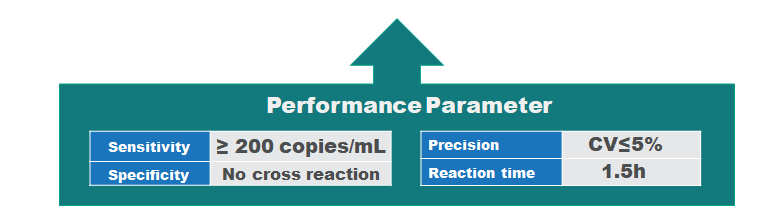
Performance parameter
SPECIFICATION
| Sample type | nasopharyngeal swab, oropharyngeal swab, deep sputum |
| Report time | 15h |
| Storage | -15°C |
| Validity period | 12 months |
| Specifications | 48 tests/box |

 Request
Request
Applicable Instrument

CATALOGS
 2 pages
2 pages