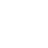
* TB-IGRA
This product is used to qualitatively detect the specific T cell immune response of Mycobacterium tuberculosis in human fresh peripheral venous anticoagulant blood in vitro.
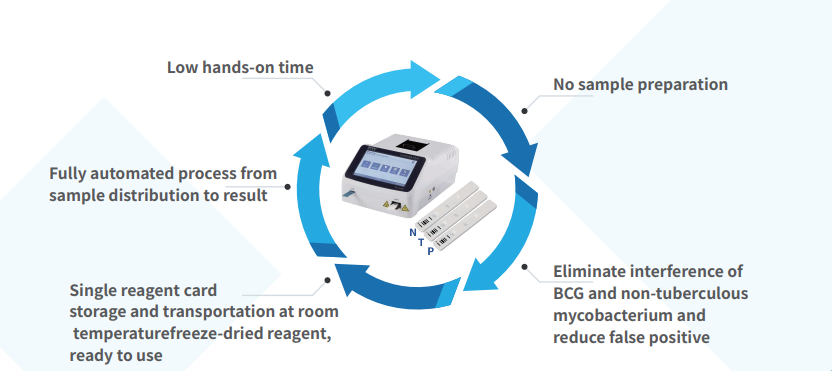
Single reagent card, can be stored and transported at room temperature; frozen intervene in culture tube
Small and easy to maintain, one-button operation, 20 minutes to report results
The results are objective and accurate, high specificity, eliminating interference from
BCG vaccine, and without the need for manual cell washing and counting
DESCRIPTION
Successfully obtained in the EU CE access certification
Passed ISO13485 certification
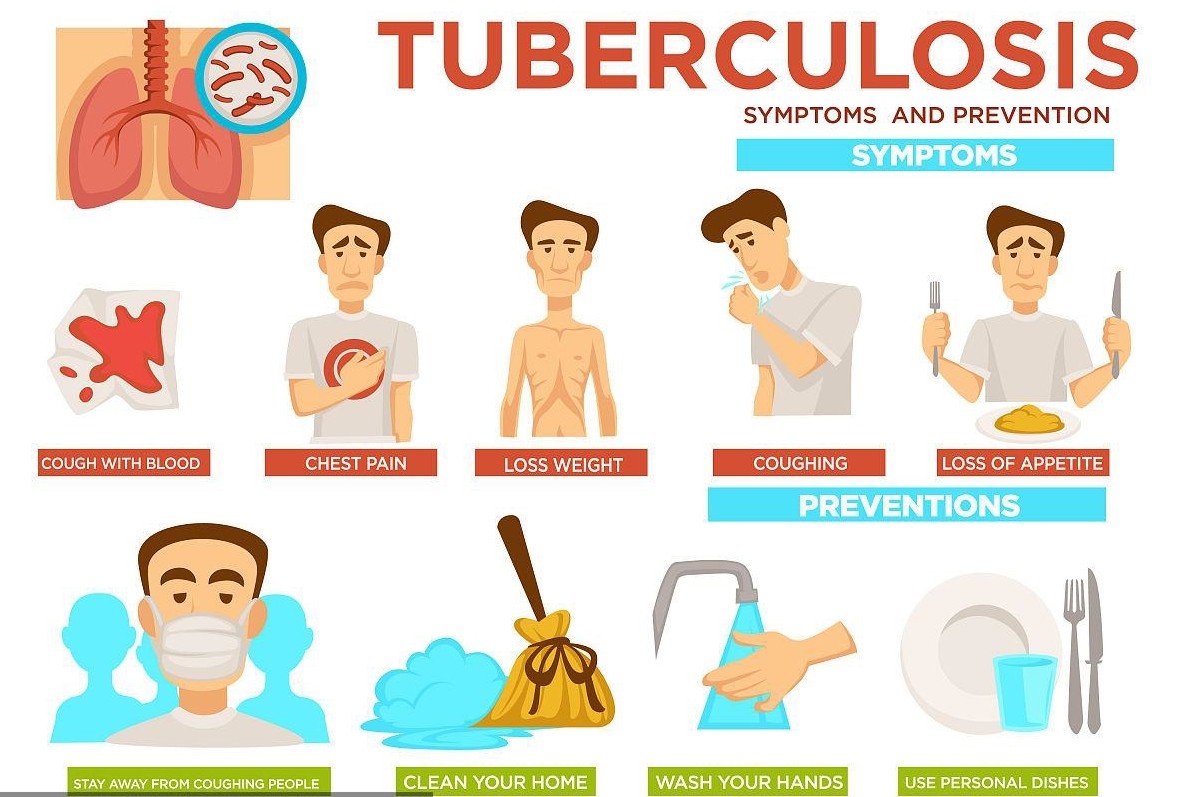
Mycobacterium tuberculosis can invade all tissues and organs of the body, but lung infection is the most common. The clinical symptoms are mainly long-term cough, sputum, hemoptysis, chest pain, dyspnea, low or high fever, fatigue, weakness, weight loss, night sweats, etc. At present, the clinical diagnosis of tuberculosis pathogens mainly adopts mycobacterial acid-fast smear staining, culture and molecular diagnosis methods.
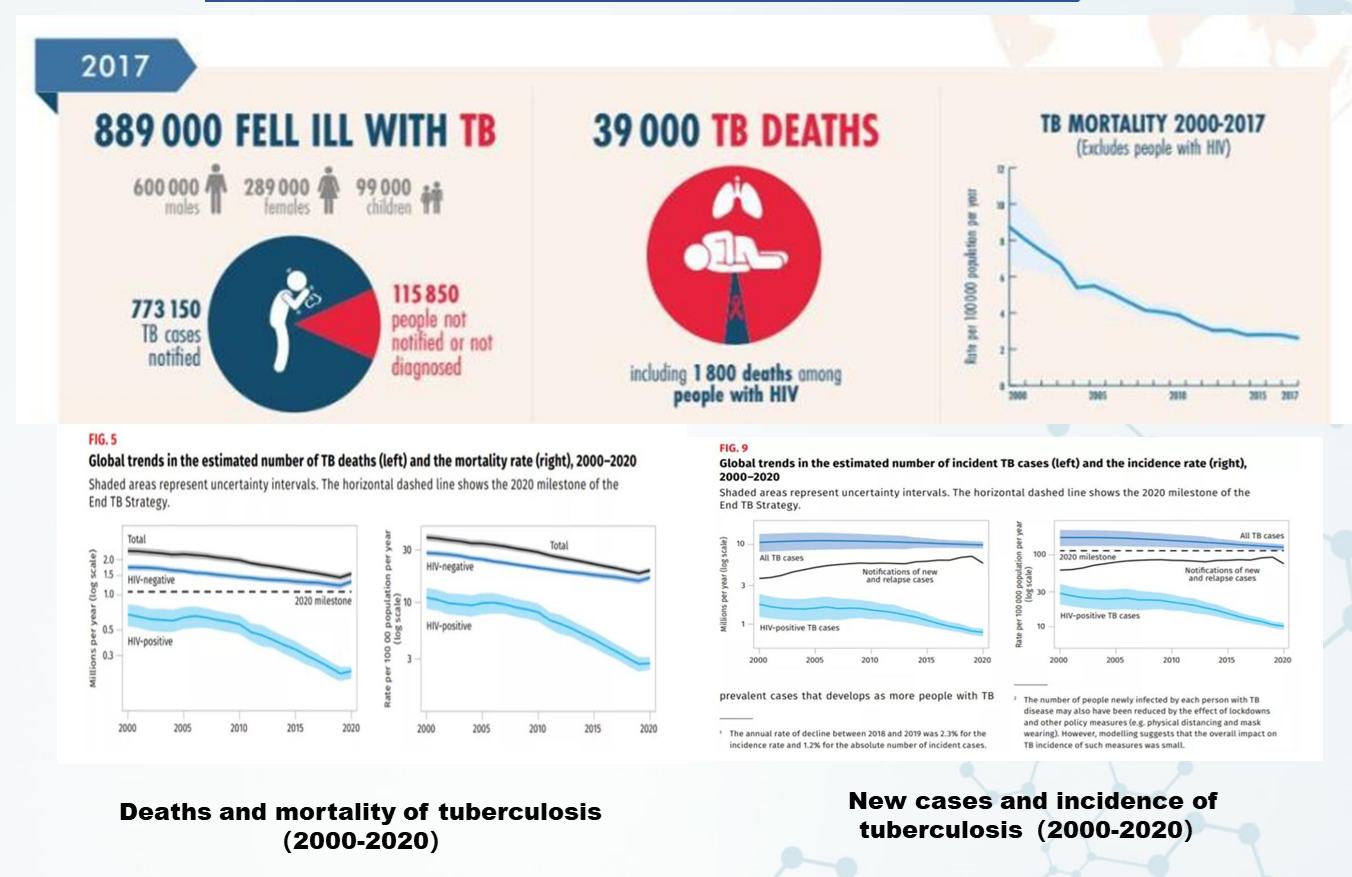
《GLOBAL TUBERCULOSIS REPORT 2020》
- Global new cases of tuberculosis in 2017 was
889000
- Tuberculosis is the killer of people living with HIV
Product advantage
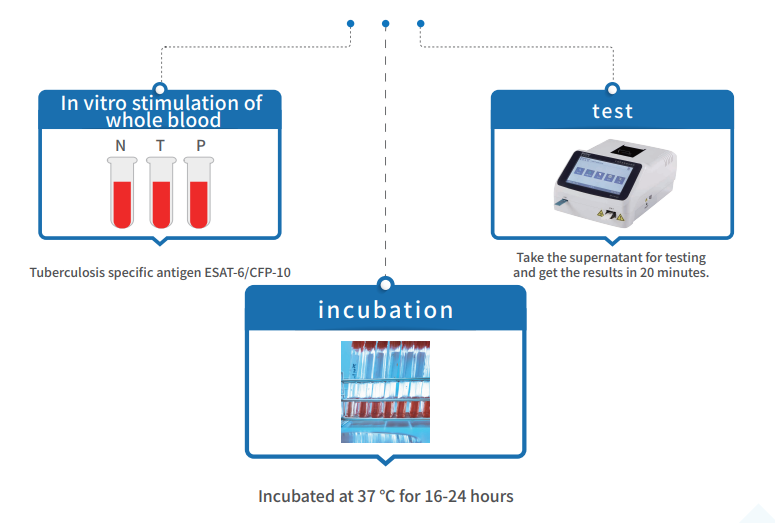
Operation steps
Clinical significance:
• Auxiliary diagnosis of tuberculosis
• Auxiliary diagnosis of extra-pulmonary tuberculosis
• Screening of latent tuberculosis for high-risk groups (biological agents/immunosuppressants, HIV patients, hemodialysis, organ transplant patients, etc.)
Applicable departments:
Tuberculosis/respiratory department, infectious/infectious department, rheumatology department, gastroenterology department, pediatrics, reproductive center
SPECIFICATION
| Sample type | Fresh lithium heparin anticoagulated whole blood |
| Report time | 20min |
| Storage | 4-30℃, sealed and kept away from light and dry, the validity period is 12 months |
| Specifications | 50 tests/box |

 Request
Request
Applicable Instrument

CATALOGS